Background and Methods:FLT3-internal tandem duplication (FLT3-ITD) is a type of mutation present in approximately 20-30% of patients with acute myeloid leukemia (AML) and is associated with poor prognosis. We performed a comprehensive genome profiling assay in patients with relapsed and refractory (R/R) AML, using the Foundation One Heme (F1H) panel, as a part of hematologic malignancies (HM)-SCREEN-Japan 01 (UMIN000035233), an actionable mutation profiling multicenter study. In this study, we analyzed the high frequency of FLT3 mutations in patients with R/R AML and found several patients who were positive for FLT3-N676K, a subclonal gene without concurrent ITD. Moreover, clonal evolution was observed in most patients who received kinase inhibitors, suggesting that mutations in signaling pathways downstream of FLT3 and activation of alternative pathways contribute to resistance mechanisms after FLT3 inhibitor treatment. Upon treatment failure during gilteritinib or quizartinib monotherapy, we can switch to another FLT3 inhibitor treatment in Japan. However, few reports have investigated clonal evolution after multiple FLT3 inhibitor treatment failure, and the mutational resistance profile remains unknown. Thus, we extended our investigation to examine clonal evolution during the progression of leukemia harboring FLT3-ITD and tyrosine kinase domain mutation (TKD) mutations. In this study, we performed serial comprehensive genome profiling analyses to evaluate time-dependent changes in genomic profiles of patients receiving the FLT3 inhibitors gilteritinib, and quizartinib in AML.
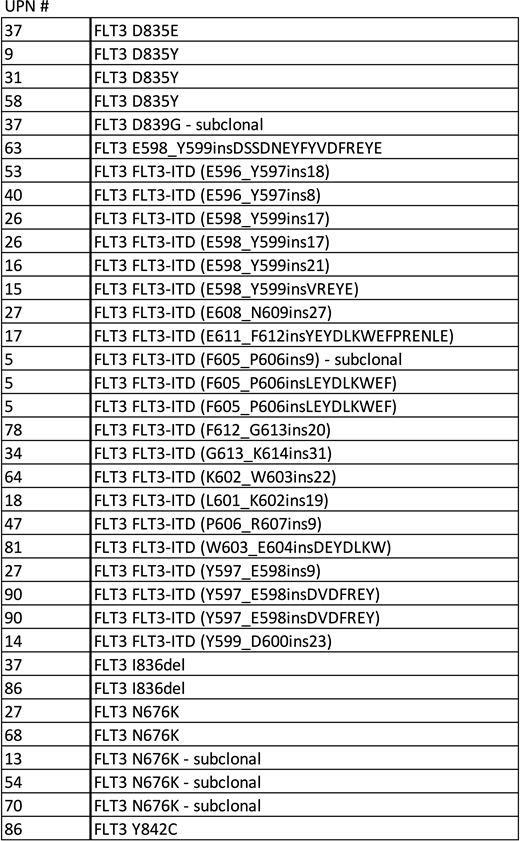
Results: This study was initiated in January 2019, and 91 patients were recruited by March 2020. The median turnaround time between sending specimens and receiving results was 15 days (9 to 56 days). In this study, a higher incidence of FLT3 mutations was observed in patients with AML (28.6%, 26/91), and those with relapse/refractory (R/R) AML (64.8%, 59/91), FLT3-ITD (20.3%, 12/59), and FLT3-TKD (15.3%, 9/59), than previously reported in newly diagnosed patients. Particularly, FLT3 mutations were much more frequently observed in patients with R/R AML (35.6 %, 21/59), whereas those with newly diagnosed AML unfit for standard treatment (15.6 %, 5/32). Furthermore, FLT3-TKD mutations were found in 10 patients, who were potential candidates for treatment with FLT3 inhibitors, such as gilteritinib. The N676K mutation within the FLT3 tyrosine kinase domain 1, which is not detectable through conventional mutational analyses, was observed using a multiplex polymerase chain reaction in 19.2% (5 of 26) of patients who were FLT3mutation-positive, including those with subclonal mutations. Moreover, patients with RUNX1 mutation were present in this cohort, and this finding supports previous reports showing the association between the core binding factor protein complex and FLT3 N676K mutation in leukemia. Interestingly, FLT3-ITD mutations usually occur in the juxtamembrane domain, but we found three patients with other abnormalities, including ITDs in the tyrosine kinase domain, which are associated with poor prognosis. Meanwhile, during FLT3 inhibitor treatment, the resistant clonal expansion was observed due to activation of alternative pathways such as NRAS pathway or acquired FLT3 mutation. Not only activating these alternative pathways, but the cases in which TKD point mutation was added to FLT3-ITD, or new mutations were acquired without the additional mutation site among the cases showing FLT3 inhibitor resistance as the treatment progressed.
Conclusions: We conclude that F1H mutational analyses for R/R AML harboring FLT3-ITD/TKD mutations may reveal novel therapeutic targets that are sensitive to FLT3 inhibitors. Moreover, improved biomarker analysis methods for detecting additional FLT3 alternations, like FLT3 N676K, could guide patient selection for the most suitable anti-FLT3 therapies. Furthermore, serial comprehensive genome profiling analysis at the time of AML progression, especially after tyrosine kinase inhibitor treatment, will provide valuable information for clinical decision-making.
Shibayama:Fujimoto: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Otsuka: Honoraria; Kyowa Kirin: Honoraria; Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Daiichi Sankyo: Honoraria; Nippon Shinyaku: Honoraria, Research Funding; Sumitomo Dainippon: Honoraria, Research Funding; Merck Sharp & Dohme: Research Funding; Takeda: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Taiho: Research Funding; Shionogi: Research Funding; Teijin: Research Funding; Astellas: Research Funding; Sanofi: Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundi Pharma: Honoraria. Yamauchi:Otsuka: Research Funding; Ono Pharmaceutical: Honoraria; Chugai: Honoraria; Abbie: Research Funding; Solasia Pharma: Research Funding; Astellas: Research Funding; Daiichi Sankyo: Research Funding; Pfizer: Honoraria, Research Funding. Gotoh:Eisai: Honoraria; Alexion pharmaceuticals: Research Funding; Ono Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria; Takeda pharmaceutical: Honoraria; Taiho pharmaceutical: Honoraria; Chugai: Honoraria; Novartis: Research Funding. Yamamoto:Chugai: Consultancy, Honoraria, Research Funding; Eisai: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; Sumitomo Dainippon: Honoraria; Stemline Therapeutics: Consultancy; Otsuka: Consultancy, Honoraria, Research Funding; Pfizer: Honoraria; IQIVA/HUYA: Honoraria; HUYA: Consultancy; IQIVA/Incyte: Research Funding; Solasia Pharma: Research Funding; SymBio: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Yakult: Research Funding; Zenyaku: Research Funding; Astra-Zeneca: Consultancy, Research Funding; Bayer: Research Funding; Bristol-Myers Squibb: Honoraria; Aichi Cancer Center: Current Employment; Kyowa Kirin: Honoraria; Meiji Seika Pharma: Consultancy, Honoraria; Mochida: Honoraria; Gilead Sciences: Research Funding; Daiichi Sankyo: Consultancy; MSD: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Ono: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Fujishima:Pfizer: Speakers Bureau. Takahashi:Novartis Pharma KK: Honoraria, Research Funding; Pfizer Japan Inc.: Honoraria, Research Funding; Bristol-Myers Squibb Company: Honoraria. Usuki:Apellis: Research Funding; Alexion: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Chugai: Research Funding. ONO:Astellas Pharma Inc.: Honoraria; TAIHO PHARMACEUTICAL CO., LTD.: Research Funding; Mundipharma K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Novartis Pharma KK: Honoraria; Celgene: Honoraria, Research Funding; Bristol-Myers Squibb Company: Honoraria; Pfizer Japan Inc.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; ONO PHARMACEUTICAL CO., LTD.: Honoraria, Research Funding; Takeda Pharmaceutical Company Limited.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria, Research Funding; DAIICHI SANKYO COMPANY, LIMITED.: Honoraria; Janssen Pharmaceutical K.K: Honoraria; Eisai Co., Ltd.: Honoraria. Kuroda:Sysmex: Research Funding; Otsuka Pharmaceutical: Honoraria, Research Funding; Astellas Pharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Ono Pharmaceutical: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Asahi Kasei: Research Funding; Shionogi: Research Funding; Dainippon Sumitomo Pharma: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Janssen Pharmaceutical K.K: Consultancy; Eisai: Honoraria, Research Funding; Fujimoto Pharmaceutical: Honoraria, Research Funding; Taiho Pharmaceutical: Research Funding; Bristol-MyersSquibb: Consultancy, Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; MSD: Research Funding. Izutsu:Incyte: Research Funding; Eisai: Research Funding; AstraZeneca: Research Funding; Abbvie pharmaceuticals: Research Funding; Chugai: Research Funding; Novartis: Research Funding; Celgene: Research Funding; Symbio: Research Funding; Solasia: Research Funding; Janssen: Research Funding; Yakult: Research Funding; HUYA Japan: Research Funding; Sanofi: Research Funding; Daiichi Sankyo: Research Funding; Bayer pharmaceuticals: Research Funding; Ono Pharmaceutical: Research Funding. Minami:Novartis Pharma KK: Honoraria; Pfizer Japan Inc.: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb Company: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal